Global Implications for Pioneering Treatment in Menzies Premature Baby Study
Global Implications for Pioneering Treatment in Menzies Premature Baby Study
The findings of the OPTIMIST-A trial, a multicentre clinical trial of premature infants with respiratory distress syndrome, were today published online in the prestigious Journal of the American Medical Association (JAMA). The study was led by Prof. Peter Dargaville at the University of Tasmania’s Menzies Institute for Medical Research and was conducted in thirty-three neonatal intensive care units in eleven countries.
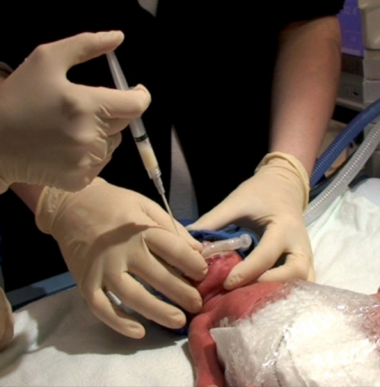
485 premature babies around the world were enrolled in this study in the first hours of life. The trial then examined the effect of delivery of surfactant by the novel Hobart method, developed by Prof. Dargaville and his team at the Royal Hobart Hospital. The aim was to improve upon the current standard of care, seeking to limit the development of bronchopulmonary dysplasia (BPD), a chronic disease of the lung that can have lasting effects on the lives of preterm infants.
The main findings of the study were that, while the rate of survival was not different in the statistical analysis, the likelihood of developing BPD was reduced from 45% to 37%. Further, babies receiving minimally-invasive surfactant therapy were half as likely to need to be mechanically ventilated with a breathing tube in the windpipe in the first 3 days and the need for oxygen therapy at home was reduced by one third.
Prof. Dargaville said it was a great privilege and very exciting that what began as an idea has become a therapy of proven benefit for premature infants.
“The findings of the study have absolutely cemented the idea that using a minimally-invasive technique to give surfactant can give these babies an advantage. The 8% difference between the groups in the rate of bronchopulmonary dysplasia, the chronic lung disease of the premature infant, is an important effect.”
Whether this healthier start to life can have a lasting impact is being examined in an innovative follow up study in which parents of infants involved in the trial are completing an online questionnaire asking about their child’s health and well-being in the first two years after the early birth.
The results of the OPTIMIST-A trial are being presented at neonatal conferences world-wide, and the Hobart method has gained traction in many neonatal intensive care units across the globe.
“Our results suggest that the use of the Hobart method for giving surfactant on day one will translate into a healthier start to life for premature infants around the world,” Prof. Dargaville concluded.